FDA clears Anterion upgrade
Heidelberg Engineering has announced the epithelial thickness module for its anterior segment imaging platform Anterion has received FDA clearance.
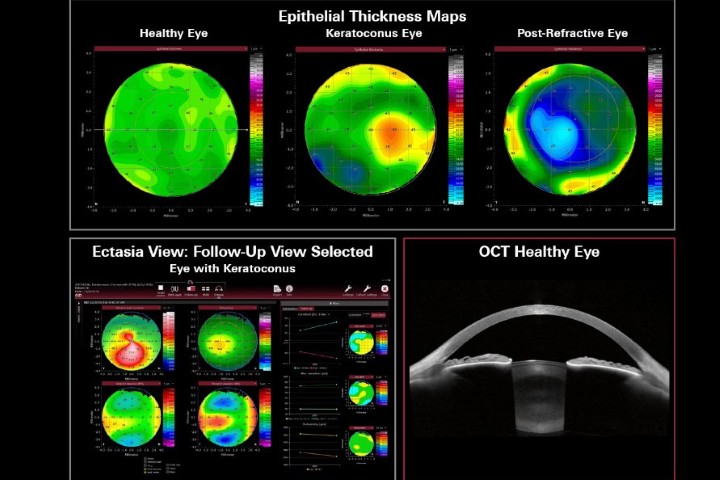
With detailed parameters and colour maps, the module is designed to support refractive surgery planning, assess ocular surface evaluation, aid in corneal ectasia assessment and assist in a variety of other cornea diagnostics, said a company statement. “This module equips eyecare professionals with advanced thickness mapping and data insights to evaluate both the epithelial and stromal structures.”
“From the beginning, our vision has been to provide precise data and reproducible measurements that allow clinicians to make informed decisions, enhance patient care and streamline workflows. This latest FDA clearance is a key milestone in fulfilling that mission,” said Ram Liebenthal, general manager of Heidelberg Engineering USA.
Anterion now also includes ‘Ectasia View’, a toolset designed to help clinicians assess and track ectatic changes in the cornea, including conditions such as keratoconus. This feature integrates corneal data from multiple visits into a single dashboard, offering a quick overview of essential tomographic maps and parameters, said the company.