FDA clears EndoArt study
The FDA has granted investigational device exemption approval to EyeYon Medical to begin a clinical study of EndoArt, the company’s synthetic endothelial implant for chronic corneal oedema.
The Israeli company said the study will be the first US-based evaluation of EndoArt, which is designed to replace endothelial function without the need for donor tissue. The trial will be led by Dr Francis Mah, director of cornea and external disease at Scripps Clinic Medical Group in California, with at least 10 surgeons participating across multiple US centres. At the time of writing, study design details and timelines had not been disclosed.
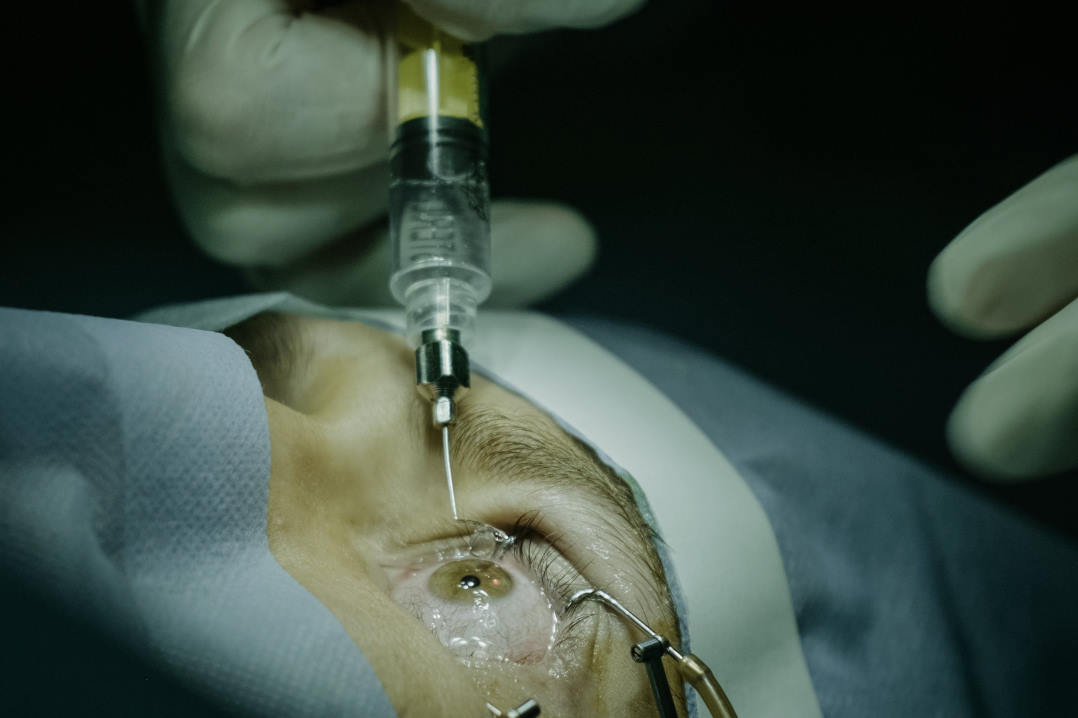
EyeYon describes EndoArt as a biocompatible acrylic hydrophilic implant that attaches to the posterior cornea to prevent fluid ingress and reduce corneal oedema. It has a three-year shelf life and is implanted using a minimally invasive, Descemet’s membrane endothelial keratoplasty-like technique, typically taking 30–40 minutes under sedation.
EndoArt received CE marking in 2021 for chronic corneal oedema and entered commercial use in 2023. EyeYon reported more than 400 implants by September 2024 and has since surpassed 800 worldwide.
Published clinical data include a small retrospective case series with six-year follow-up, reporting sustained reduction in corneal oedema and a favourable safety profile in selected patients. EyeYon said the US study will further assess safety and performance in a broader clinical setting.